As one of the essential products of nylon materials, nylon-6 is widely used in the nylon fiber, engineering plastics and film industries. Cyclohexanone oxime is an important precursor for the synthesis of nylon-6, and more than 90% of it is produced by the traditional cyclohexane-hydroxylamine method (Figure 1a) in the world. However, this method needs hazardous hydrogen and corrosive sulphuric acid, which have potential risks including hidden danger, high costs and environmental pollution. Researchers have therefore worked to develop alternative production strategies for cyclohexanone oxime.
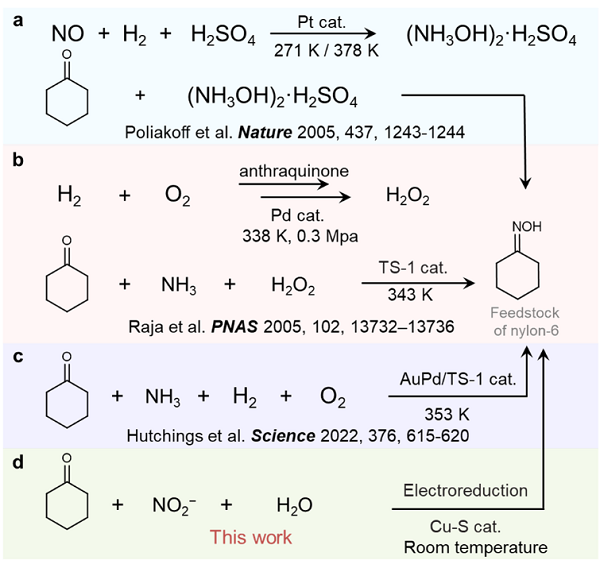
Currently, cyclohexane-ammonia oxidation, one of the most promising alternative production methods for cyclohexanone oxime, has already been used in industrial production. In this method, H2O2 is used as an oxidant to oxidise NH3 in situ to produce hydroxylamine (NH2OH), which is then condensed with cyclohexanone to produce cyclohexanone oxime (Figure 1b). However, this method requires the use of H2 and an excess of H2O2, which is of high cost and likely to corrode devices. Recently, Hutchings et al. developed a method for the in situ production of H2O2 using H2 and O2 to react with cyclohexanone to synthesize cyclohexanone oxime (Figure 1c). This method does not require excessive H2O2 and is more economical, but still requires precious metal catalysts, H2 and high temperature. Therefore, it is important to develop a green, mild and efficient strategy for the synthesis of cyclohexanone oxime.
Recently, postdoctoral Wu Yongmeng from Zhang Bing’s research group of the School of Science developed a green electro-synthesis method for cyclohexanone oxime (Figure 1d). Under ordinary temperature and pressure, the method uses nitrite as the nitrogen and water as the hydrogen, avoiding the high temperature and pressure conditions of existing methods as well as the use of precious metal catalysts and sulphuric acid/hydrogen peroxide. Cyclohexanone oximes were obtained in yields up to 92% and selectivity up to 99%. Therefore, both economic and technical analysis show that the method is profitable. The study not only provides a green, gentle and sustainable strategy for the production of cyclohexanone oxime, but is also expected to provide new methods for other industrial processes involving hydroxylamine reactants. The related results were published in Nature Communications.
For more information, please refer to:https://doi.org/10.1038/s41467-023-38888-6
By School of Science
Editor: Sun Xiaofang






