The Institute of New-Energy Materials, Tianjin Key Laboratory of Composite and Functional Materials of Tianjin University has recently cooperated with the Key Laboratory of Micro- and Nano-scale Boron Nitride Materials of Hebei Province at Hebei University of Technology, and has achieved a significant progress in cubic boron nitride (c-BN) synthesis. The research paper “Photochemical Synthesis of Ultrafine Cubic Boron Nitride Nanoparticles under Ambient Conditions” was published in Angewandte Chemie International Edition, one of the top chemistry journals, and was elected as a hot paper. (DOI: 10.1002 / anie. 201502023).
Cubic boron nitride is a super-hard material whose hardness increases sharply with the decrease in size. It is widely used in tool cutting materials. However, cubic boron nitride remains stable under high pressure, thus the synthesis of it is usually conducted under high pressure (several tens GPa) and high temperature (1000-2000 K). Currently, the minimum size of a synthetic cubic boron nitride particle is 14 nm.
Professor Du Xiwen’s research team at Tianjin University obtained many pioneering achievements in the field of laser synthesis of materials. He set up a theory on controllable synthesis of nanostructures by long-pulse-width laser, and produced various novel nanostructures, which was highlighted by Nature China.
(Angew. Chem. Int. Ed. 2015, 54, 1, Nat. Commun. 2013, 4, 1695, Angew. Chem. Int. Ed., 2011, 123, 4185, J. Am. Chem. Soc. 2010, 132, 9814)
Professor Tang Chengchun’s team at Hebei University of Technology successfully achieved green synthesis, performance research, and application development of new materials of boron nitride. Based on their respective strengths, both teams cooperated in the study of laser synthesis of cubic boron nitride and tried a variety of process routes and material systems. Finally using laser irradiation of ammonia borane solution, ultrafine (3.5 nm) cubic boron nitride nanoparticles were synthetized under normal temperature and pressure.
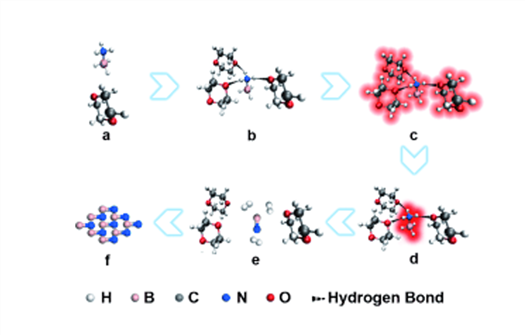
Studies have found that an ammonia borane molecule could form a basic unit with three polar solvent molecules. This basic unit can capture four laser photons. It is possible that the energy is transferred to the central ammonia borane molecule through the hydrogen bonds, and breaks the bond to complete dehydrogenation. Using a strong laser, large amounts of boron nitride molecules will be produced in solution, which will cause the explosive nucleation of boron nitride particles and then obtain ultrafine boron nitride particles. Large radius of curvature of ultrafine nanoparticles can produce additional surface pressure, which can make the cubic phase stable at room temperature. Finally it will get the cubic boron nitride particles.
This research has achieved the following breakthroughs. Firstly, for the first time it reported the synthesis of cubic boron nitride at room temperature and normal pressure, and the synthesis speed is extremely fast, taking 10 minutes in the entire process. Secondly, it synthetized for the first time ultrafine cubic boron nitride nanoparticles with a size of 3.5 nm, and with the expectation that its hardness should reach its peak. Thirdly, it quickly realized the complete dehydrogenation of ammonia borane, providing a new technology for quick hydrogen desorption of hydrogen storage materials. This research is supported by the Natural Science Foundation of China (51041006, 51402084, 21103224).






