A new catalyst CoO/CoSe2 for water electrolysis has been recently synthesized by the research team under the guidance of Professor Zhang Bing from the School of Science at Tianjin University. As a mosaic nanocomposite, this catalyst can spontaneously catalyze the production of hydrogen and oxygen in neutral environments with high efficiency and stability. The hydrogen is produced around the negative pole through water reduction, while the oxygen around the positive pole through water oxidation. This new achievement now has been published in the international journal Advanced Science.
Hydrogen production by water electrolysis is an effective technology to produce clean and sustainable energy. The efficiency of electrical energy into hydrogen energy depends on the quality of catalyst for water electrolysis. Currently, noble metals like platinum and ruthenium dioxide perform well as the catalyst for water electrolysis. Unfortunately, this kind of catalyst is inapplicable to large-scale industrial production due to the scarcity and high cost. In recent years, many catalysts have also been successfully synthesized by scientists around the world. However, the complicated synthesis methods, the bad conductivity and stability of these catalysts have hampered their widespread and large-scale application.
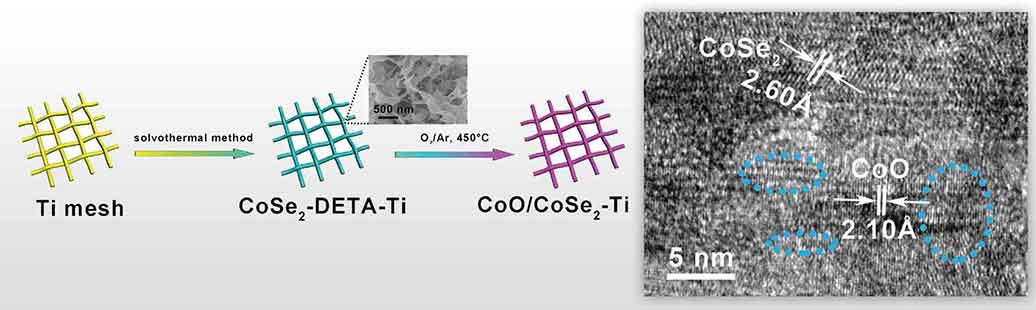
To improve these deficiencies, Professor Zhang guided the research team to successfully develop a new catalyst for water electrolysis which is cheap and easy to synthesize. They immobilized the super-thin nano material CoSe2-DETA on the titanium mesh substrate, which constitutes a self-standing catalyst with high conductivity. Through this method, the CoSe2 with metallicity was partially oxidized on the surface and converted into CoO embedded on the surface of CoSe2. Then they obtained this nanocomposite catalyst CoO/CoSe2 for water electrolysis.
The development of the new catalyst has a lot of merit. For instance, it has high stability as CoO and CoSe2, these two solidly mixed substances, can be directly used in the electrochemical reaction to avoid falling off the surface of poles. Compared with noble metals, selenium and cobalt are much cheaper in terms of cost. It is also highly efficient because the super-thin structure and titanium mesh substrate give it the ability to increase effective contract. Meanwhile, the metallic CoSe2 and conductive titanium mesh substrate immensely enhance the conductivity of the catalyst for they speed up the transmission rate of electrons. The newly produced CoO can effectively adjust the electronic structure of the CoSe2, which strengthens the performance of the catalyst in catalyzing the productions of hydrogen and oxygen in neutral environments.
Professor Zhang pointed out that the new catalyst is promising prospect for industry application as it can be synthesized cheaply and easily. It also can produce more hydrogen with less energy consumption in neutral environments. Therefore, this synthesis method provides new ideas for the syntheses of other innovative composites.






