Recently, scientists revealed the dynamic process and mechanism of interfacial structure evolution in CuAu alloy under reaction conditions as well as the synergy mechanism between Cu and Au under the water gas shift reaction (WGSR) in atomic scale through multiple research methods including the aberration corrected environmental transmission electron microscopy (AC-ETEM), the density functional theoretical (DFT) calculation, the ab initio molecular dynamics simulation (AIMD) and the in-situ diffusion reflectance infrared Fourier transform spectroscopy (DRIFTS). The relevant research work has been published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on 1st June, 2022.
The research was corporately studied by Prof. Langli Luo’s group from the Institute of Molecular Plus of Tianjin University (TJU), Prof. You Han’s group from the School of Chemical Engineering and Technology (TJU) and Prof. Jing Xu’s group from the School of Chemical Engineering (ECUST).
Bimetallic catalysts often show improved catalytic performance compared with their single-element counterparts, which is usually attributed to the so-called “synergy effects” of two metal elements. Meanwhile, heterogeneous catalysts often exhibit dynamic structural and chemical changes on catalysts surface during reaction, further complicating the identification of the origin of “synergetic effects”. To understand how different metal elements spatially and temporally evolve for a working bimetallic catalyst upon reaction to decipher the reaction routes and mechanisms, the dynamic evolution mechanism of the CuAu surface structure and the synergy catalytic mechanism of Cu and Au were explored in depth in this paper based on their previous work (Angew. Chem. Int. Ed. 2020, 59, 2505; J. Am. Chem. Soc. 2020; 142, 8, 4022, Phys. Rev. Lett. 2020. 125, 156101, etc.).
In this paper, researchers observed the interfacial structural evolution of CuAu (atomic ratio of Cu/Au=9:1) in-situ at 300 oC when exposed to CO and water vapor respectively with different gas pressure in AC-ETEM. A rough “saw-teeth” structure appeared at CuAu(110) surface due to surface segregation of Au atoms under low CO pressure, while more vigorous surface reconstruction was observed with increasing CO pressure. In water vapor atmosphere, surface adsorption and subsurface diffusion of H2O (or its dissociated species such as O or OH), surface oxide formation were observed gradually with the increasing of H2O pressure (Figure 1).
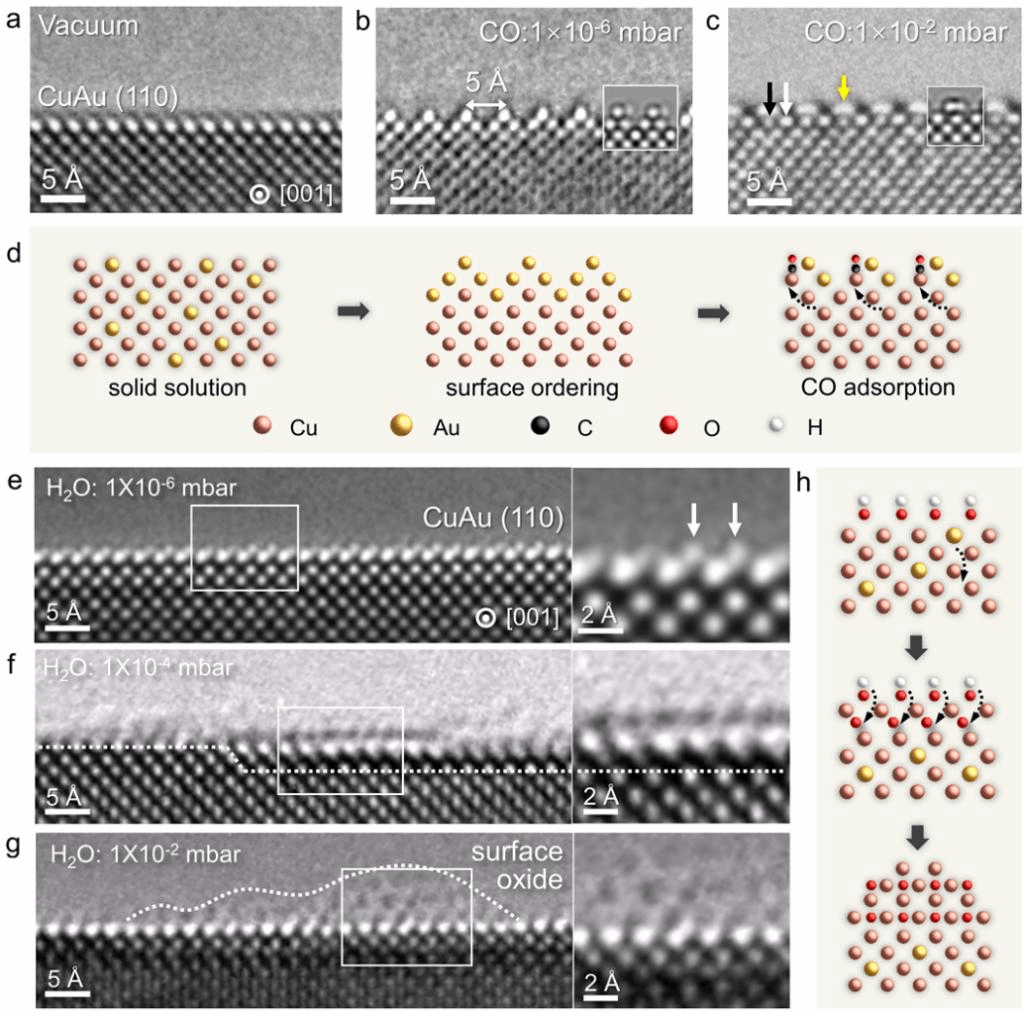
Figure 1. Structural evolution of CuAu surface when exposed to CO and water vapor, respectively.
DFT calculation was employed to find the possible adsorption sites of CO and H2O on CuAu surface to understand the surface reconstruction phenomenon results from gas adsorption (Figure 2). Researchers designed three different structure models (Cu9Au1, Cu3Au1, Cu3Au1_rgh) for calculations based on the surface segregation of Au and the rough surface under CO atmosphere. The DFT calculation results could reasonably explain the observed phenomenon, i.e., CO is preferred to adsorbed on Au atom at the top of the rough surface to form the elongated lattice points and H2O is preferred to adsorbed on the bridged Cu sites to form smaller lattice points between the CuAu lattice points.
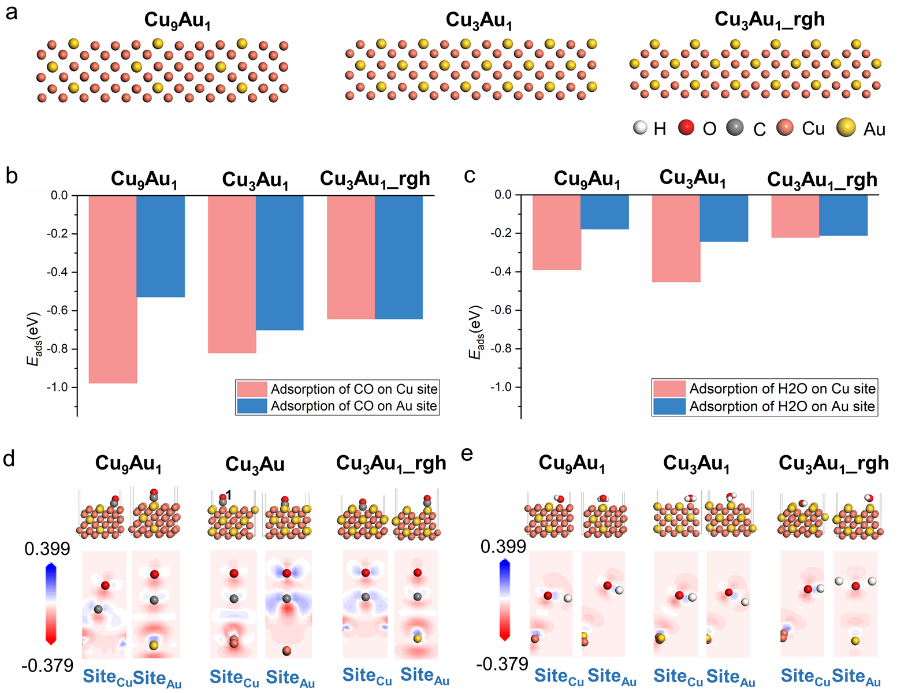
Figure 2. Adsorption of CO and H2O on CuAu surface through DFT calculation
According to the experimental phenomenon and the theoretical calculation results shown above, the authors anticipate a competitive adsorption and reaction process of CO and H2O on CuAu surface under WGSR conditions (300 ℃, CO:H2O=1:1). Hence, in-situ ETEM experiments were conducted and periodic surface activation phenomenon was observed under WGSR conditions. With increasing pressure of gas mixture, the atomic structure of the activated surface was evolved from a periodic wavy atomic column to a periodic elongated and additional atomic column and finally forms a stable periodic surface structure (Figure 3). AIMD simulation was employed to investigate the reaction process on activated CuAu surface that the pre-adsorbed CO and dissociated H2O species interact and form CO2 then desorb from CuAu surface. The reaction route proposed by AIMD simulation could be supported by the in-situ DRIFTS of CuAu alloy under WGSR conditions.
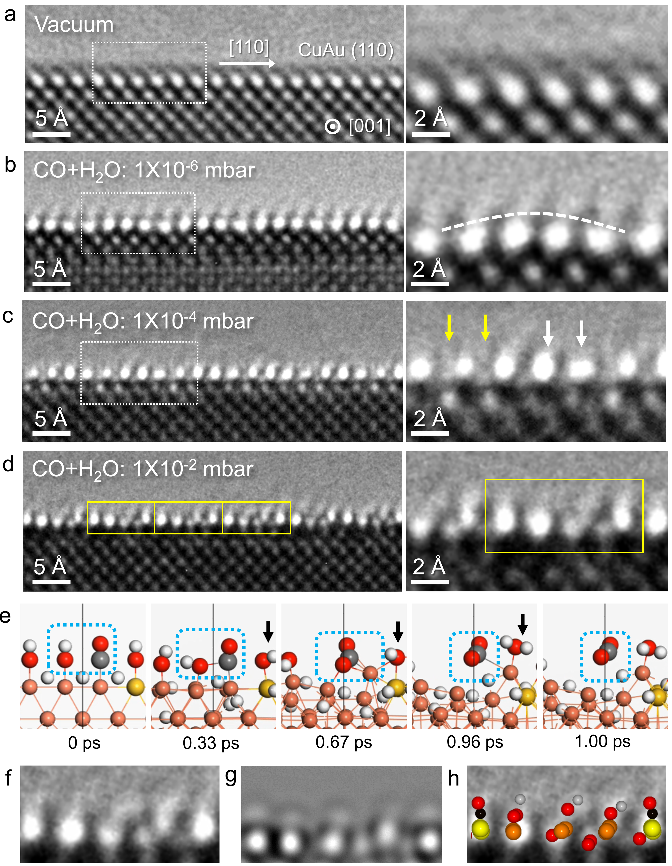
Figure 3. Synergetic activation of the CuAu surface under WGSR conditions.
Furthermore, the reaction routes when CO adsorbed on different Cu sites on CuAu surface and O adsorbed on CuAu surface were investigated systematically through AIMD simulations. The results show that WGSR can be promoted when CO is adsorbed on Cu sites adjacent to Au (Figure 4), further demonstrating the synergistic relationship between Cu and Au under WGSR.
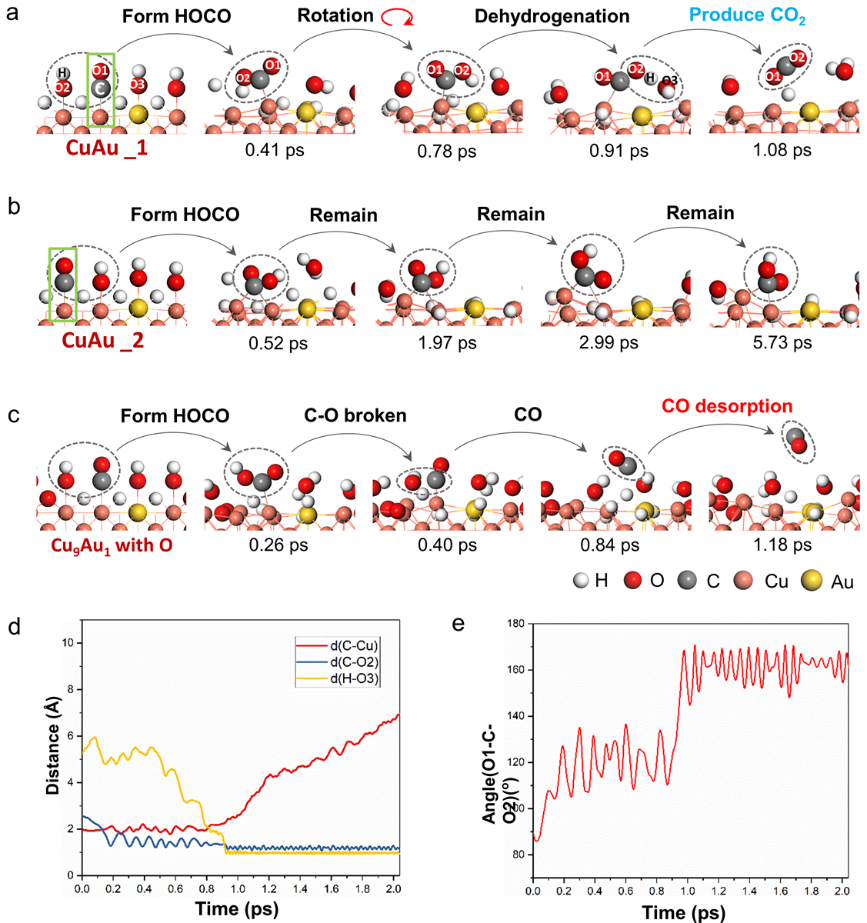
Figure 4. The dynamic reaction process of the CO and dissociated H2O species adsorbed on CuAu surface analyzed through AIMD
In this paper, AC-ETEM was employed to investigate the periodic surface reconstruction on CuAu surface under WGSR conditions. The local environment on CuAu surface, as discussed by DFT and AIMD calculations, plays a significant role in adsorbing reactants and intermediate during the WGSR process. The synergy mechanism between Cu and Au in WGSR was also revealed from the dynamic evolution of CuAu surface. The activation of local environment on catalyst surface by reactants observed here may help to build a theoretical foundation on the directed design and precise control of high-performance catalysts.
Dong Zejian (Postdoc, Institute of Molecular Plus, TJU), Nian Yao (PhD candidate, School of Chemical Engineering and Technology, TJU), Liu Hongpeng (PhD candidate, Institute of Molecular Plus, TJU), Chen Jiacheng (PhD candidate, School of Chemical Engineering, ECUST) are listed as co-first authors of this paper. Prof. Langli Luo, Prof. You Han, Prof. Jing Xu are the corresponding authors.
Paper Link: Zejian Dong#, Yao Nian#, Hongpeng Liu#, Jiacheng Chen#, Yan Wang, Shuangbao Wang, Jing Xu*, You Han*, Langli Luo*. Revealing synergetic structural activation of CuAu surface during water gas shift reaction. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(23): e2120088119.https://doi.org/10.1073/pnas.2120088119
By: School of Chemical Engineering and Technology
Editor: Qin Mian






