Recently, Prof. Weng Zhe from Nanoyang Group in the School of Chemical Engineering and Technology published an article entitled “A Self-Regulated Interface toward Highly Reversible Aqueous Zinc Batteries” in Advanced Energy Materials (https://onlinelibrary.wiley.com/doi/10.1002/aenm.202102982). Dr. Han Daliang and master candidate Wang Zhenxing were co-first authors.
Aqueous zinc (Zn) batteries have been considered one of the most promising candidates for grid energy storage because of their inherent low cost, high and outstanding environmental friendliness. Particularly, the Zn metal anode shows great promising ascendancy in abundant natural resources, high theoretical capacity (820 mAh g−1, 5855 mAh cm−3), low redox potential (−0.76 V vs the standard hydrogen electrode), and nontoxicity. However, there are still critical issues involving severe Zn dendrite growth and side reactions that greatly limit the practical application and industrialization of aqueous Zn batteries. Therefore, it is of significant importance to suppress Zn dendrite growth and side reactions simultaneously. Since these problems occur at the electrode/electrolyte interface and are highly related to the interaction between the Zn metal anode and the aqueous electrolyte, a dynamically steady interface with a good self-regulating ability is highly desired. Unfortunately, this has significant challenges and rarely mentioned.
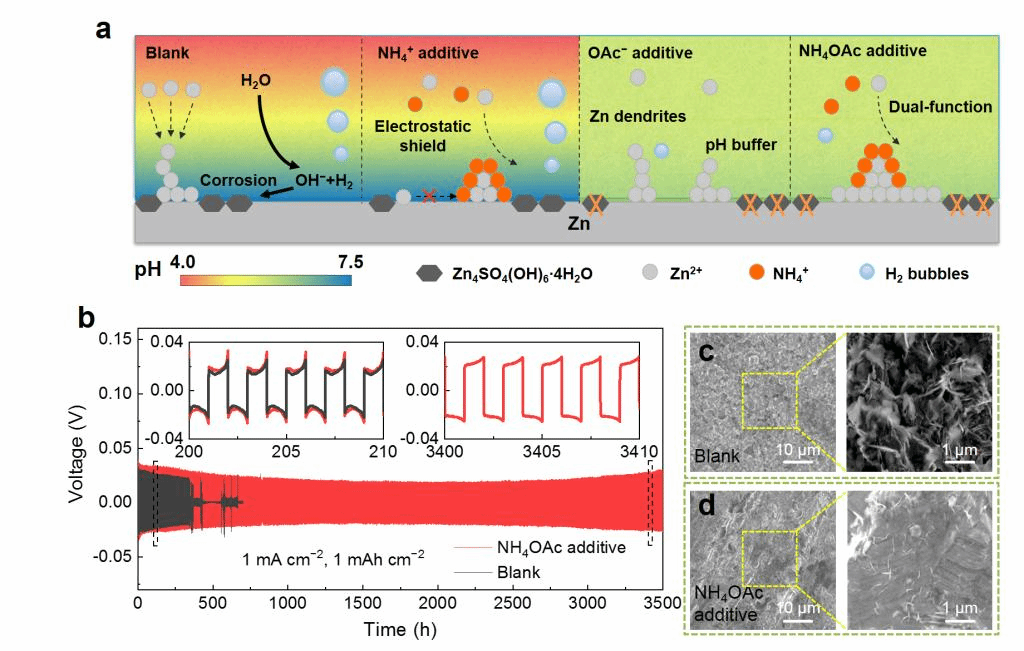
In this work, the authors reported a self-regulated Zn/electrolyte interface using a low-cost dual-functional ammonium acetate (NH4OAc) additive, which serves as both a competing cation donor and a pH buffer to maintain the interface relatively steady, thus greatly suppressing the Zn dendrite formation and side reactions simultaneously. As a competing cation, the NH4+ is demonstrated to be preferentially adsorbed on the Zn metal anode than Zn2+, which induces electrostatic shielding effect at the Zn/electrolyte interface and prevents the agglomerative Zn deposition. The OAc− plays an important role in dynamically regulating H+/OH− concentration of the electrolyte by forming the pair of acetic acid/acetate (HOAc/OAc−) and thus enables the interfacial pH to keep at an appropriate value of ≈5.14, which significantly suppresses the side reactions and reduces precipitation of insoluble by-products on the Zn metal anode.
As a result, the Zn anode sustains long cycling over 3500 h at 1 mA cm−2 and 5000 cycles at 10 mA cm−2 as well as a high Zn plating/stripping CE of 99.7% due to the synergistic effect of the NH4+ cation and OAc− anion. The modified electrolyte also enables stable cycling of a prototype full cell with an oxygen-deficient hydrated NH4V4O10 cathode.
By: School of Chemical Engineering and Technology
Editor: Qin Mian






